- Visibility 594 Views
- Downloads 67 Downloads
- Permissions
- DOI 10.18231/j.ijpp.2021.008
-
CrossMark
- Citation
Impact and overview of track and trace system and serialization in pharmaceutical industry
- Author Details:
-
Dhavalkumar Patel *
-
Dev Kumar Singh
-
Birajuben Patel
-
Chetan Borkhataria
Abstract
An alleviation of patient wellness and safety, the pharmaceutical ecotechnology play crucial role by eliminating the threat of counterfeit drugs, medicines and drug adulteration. The numerous issue of supply chain such as production of inferior copy of branded product with label of the original brand name, the risk of putting wrong product into distribution, unauthorized distribution of branded product and lack of consistency across countries are now a days creating challenges for pharmaceutical manufacturers worldwide. The regulatory authorities of countries and pharmaceutical industries believe that serialization and product tracking could be the ultimate solution for these worldwide issues. Serialization system has the capacity to find out current and past locations of the product through entire supply chain. Hence, due to urgent necessity in today’s competitive environment, the implementation of this technology has found to be more effective to enhance counterfeit prevention, pilferage reduction and targeted recalls as well as improve security, visibility, synchronization and supply chain efficiency.
Introduction
A counterfeit medicine is the fake medicine which is mislabeled intentionally and fraudulently with respect to original brand or generic product. The products contain wrong or no ingredients or insufficient ingredients or correct ingredients but wrong dose or may be contaminated or fake packaging.[1], [2]
Current scenario of global pharmaceuticals industry depicted that they face problems of theft, counterfeiting, diversion and false returns to the manufacturers. The World Health Organization (WHO) reported that counterfeit drugs/medicines are near about 1% of the total distribution in developed countries while 30% to 40% in developing countries. This kind of counterfeiting promotes the crime worldwide.[3] In this era of worldwide development, the supply chain for genuine pharmaceutical company has grown longer, and every step in the supply chain offers another opportunity for fraudsters. The challenges facing pharmaceutical manufacturers are several in addressing the problem of the supply chain security which can be categorized into four major areas as shown in the [Figure 1] .[4] The pharmaceutical industries, governments and regulatory authorities of countries worldwide believe that counterfeiting of drugs and medicines by organized crime can be minimized significantly by implementing serialization and product tracking system.[3]
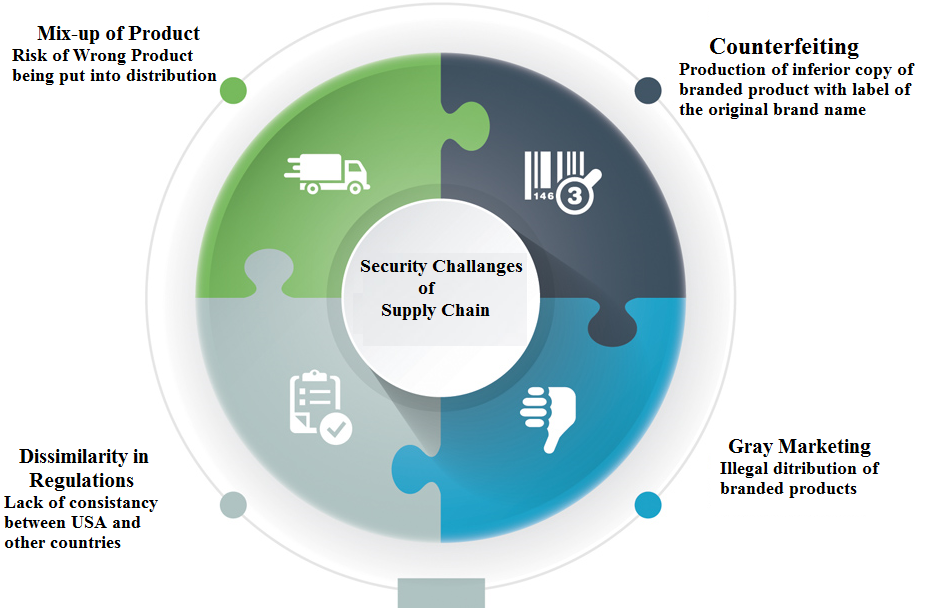
To handle this issue efficiently, there needs to be a joint effort from the pharmaceutical company, regulatory agencies, stockiest and retailers to establish a possible identification solution. A database where uniquely identifying bar code on each packaged drug product can be verified at the point of dispensing would significantly enhance the ability to track all pharmaceutical products worldwide; there is an urgent need to enhance traceability within the supply chain.[5] Serialization needs a comprehensive system and policy to track and trace the passage of prescription drugs and medicines through entire supply chain. By serialization process, it can be possible to assign unique serial number in addition to the origin, batch number and shelf life of every product. This will open the way to trace the product’s lifecycle from production, through distribution and finally to dispensation to the patients at pharmacy store or hospital.[3] In order to protect public health interests and the relationship of trust between patients and pharmaceutical product suppliers, the production and supply chain needs to be closely regulated.[6]
Worldwide Regulatory Framework: History and Recent Scenario
The concept for regulating pharmaceutical traceability date was initiated back to 2000 by the Italian ‘Bollini’ law who required the application of a specific kind of sticker containing a bar code along with serial number to each packaged unit, and the recording and archiving of data. After that, many countries in the European Union (EU) have made their legislative efforts for imposing product serialization standards including the CNK (Code National(e) Kode) in Belgium where a seven digits unique identification number was granted or the 13-digit CIP (Club Inter Pharmaceutique) code in France. Other countries, like China, India, Korea and Turkey (Placed item level serialization requirements in 2010) have actively participated in this global attempt at regulation. In November 2013, the president of the United states signed the ‘H.R. 3204 Drug Quality and Security Act (DQSA)’, a Federal law that preempts the California bill, which initially established a creation of a ‘pedigree’ for each drug product sold in the United states, and implemented item level serialization by November 2017 for all Rx (prescription) drugs sold in the States.[4], [7]
|
Overview[5] |
||
|
Act/ Rule |
Basic Requirements |
Implementation |
|
USA (US Federal) |
||
|
Drug Quality and Security Act (Track & Trace Bill) Drug Supply Chain Security Act 2013 Law 2013 |
- Securing the prescription drug supply chain framework. -Licensure standards for distributors and wholesaler and Third party logistics providers. |
10 Year Plan – 27th November 2013- 27th November 2023 |
|
USA (California) |
||
|
E-Pedigree Law Nov. 27 2013 |
Pedigree (Creation of electronic transaction history of the drug) -Source -Generic Name and Strength, Expiry, Lot Number, Transaction Date etc. -Certification |
2015-2017 |
|
European Union |
||
|
Falsified Medicine Directive (FMD) Directive 2011/62/EU European Medicine Verification System (EMVS) |
linear, 2D barcode, Radio Frequency Identification (RFI D) -barcodes printed /attached to every single pack barcodes will be checked into a database repository system by mfr. 2D data matrix barcode on the outer packaging |
2016-2019 |
|
France |
||
|
French CIP13 Coding Legislation |
-CIP13 code, Batch no. & expiry printed on each item. -Code is batch specific |
2016-2019 |
|
Japan |
||
|
Encoding of Pharmaceuticals with Japan Article Number |
Barcodes Serialization |
2008-2021 |
Serialization Blueprinting in Pharmaceutical Industry
As with any major change, the number of planning steps, strategy and decisions including myriad risks must be undertaken before implementation of any project. Initially, it is necessary for any manufacturing operations to be ready for project implementation. This includes, but not limited to current regulatory and legislative requirements; existing backup and recovery capacity of information technology system; engagement with cloud based servers; data exchange capacity with CMOs and trading partners; schedule and budgetary constraints; resources; impact on routine productivity and distribution; enough space for printing of serialized information on carton or label artwork.[8], [6]
Serialization project Implementation Plan is basically divided into four stages.
Planning stage
Identify the stakeholders (Operation, Distribution, Legal, Regulatory, Labeling, IT, Engineering, Quality, Purchasing and another relevant department) and establishment of project team.
Detail portfolio of commercial product to be serialized, package configuration (bottle, carton, case etc.), packaging line configuration including equipment and IT infrastructure.
Understand the DSCSA definitions for manufacturer, distributor, CMO, etc. and level of GS1 adoption.
Map existing packaging operation and business processes impacted by serialization.
Review technique for sharing transaction data (transaction information, history and statement).
Define process owners and departmental responsibilities of potentially separate systems.
Define existing IT capabilities.
Identify the existing technology in order to identify and quarantine illegitimate/ suspect products.
Solution design stage
Define scope, budget (pilot and rollout) and schedule.
Determine resource (internal and external) requirements.
Determine IT infrastructure for operations, backup and recovery, risk of data breach (cloud or site server).
Assess and finalize serial number management system and serialization provider (floor through enterprise).
Design scalability and flexibility into your solution.
Decide and procure necessary equipment, hardware and software.
Determine print technology and coordinate with CMOs if applicable.
Build pilot stage
Pilot line installation.
Operations and modify relevant Standard Operating Procedure (SOPs).
Training.
Performance evaluation, adjustments and optimization.
Coordinate with trading partners to make sure accurate transaction data transfer via Electronic Data Exchange (EDI).
Development stage
Determine rollout strategy for commercial product introduction to the market (once serialized, remain serialized; or convert lines but wait to implement serialization).[6], [8]
Serialization Barcoding for US FDA-Regulated Products
Demonstration of two-dimensional (2D) Bar code in product label
The GS1 DataMatrix includes the GTIN AI (01) + the serial number AI (21) + the expiration date AI (17) + the lot number AI (10) to generate the DSCSA-compliant product identifier encoded in the 2D Data Matrix bar code.

The encoded data appear as:
AI (01) + GTIN + AI (21) + Serial Number + AI (17) + Expiration Date + AI (10) + Lot Number.
Examples:

Scan of the GS1 DataMatrix symbol above yields the following data:
(01)00312345678906(21)12345678(17)180630(10)ABC123
GTIN Number [GS1 AI(01)]: 00312345678906
Represents “0”- Indicator digit, “0312345”- Company prefix, “67890”- Product code, “6”- Check digit. The properly constructed GTIN that incorporates the product’s NDC.
Serial Number [GS1 AI(21)]: 12345678
Recommends unique alphanumeric serial number of up to 20 characters
Expiration Date [GS1 AI(17)]: 180630
Recommends YYMMDD format.
Batch or Lot Number [GS1 AI(10)]: ABC123
Recommends 1 to 20 alphanumeric characters representing the batch or lot number.
Example of same label with GTIN eliminated from the human-readable text to save space (the GTIN HRI already exists on this label as associated with the UPC-A linear barcode).

Demonstration of inner packs (Bundles)
The DSCSA needs product identifiers in a 2D Data Matrix bar code on the individual saleable units while a 2D Data Matrix bar code or linear bar code on homogeneous cases. It does not need the product identifier to be encoded onto inner packs unless the pack is an individual saleable unit.
For inner packs (also known as bundles, sleeves, trays, etc.), it recommends a minimum, a 2D GS1 DataMatrix and a corresponding human-readable format.
Examples

Scan of the GS1 DataMatrix symbol above yields the following data:
(01)20312345678900(21)12345678(17)180630(10)ABC123
Demonstration of product case label (Homogeneous cases)

For DEA controlled product, it recommends not to use product description.
Scan of the GS1 DataMatrix symbol above yields the following data:
(01)50312345678901(21)123456789012(17)201231(10)123456L[9]
Conclusion
Counterfeiting in Pharmaceuticals is a critical issue addressed by regulatory agencies of several countries. This needs multiple measures in order to protect the supply chain. The enforcement of the anticounterfeit technologies is a crucial strategy taken up by various pharmaceutical manufacturers and regulatory authorities. The track and trace system and serialization come up with great importance and are acceptable worldwide among all anticounterfeit technologies. This system even plays vital role when managing a product withdrawals or recall, where accuracy and time of reporting is crucial. Moreover, recalls, drug take-backs, returns will be greatly facilitated by both track and trace and serialization. In recent years, track and trace system and serialization have shown to be an effective tool for increasing competitiveness and capacity to prevent thefts and counterfeiting while reducing the frequency and costs of the recall. Because of great importance and current need, it is highly essential to include provisions of security measures in the packaging of pharmaceuticals worldwide specially in the developing and underdeveloped country.
Source of Funding
None.
Conflict of Interest
None.
References
- . WHO/ACM/3 (2010), Report of the Situation of Counterfeit Medicines Based on Data Collection Tool Who Regions for Africa and Eastern Mediterranean. World Health Organization, Geneva, Switzerland. . . [Google Scholar]
- . U.S. Food and Drug Administration. (2019). Counterfeit Medicine. . . [Google Scholar]
- . Cognizant 20-20 Insights. (2013). Pharma Serialization: Managing the Transformation. . . [Google Scholar]
- Chatterjee B. Serialization and the Drug Quality & Security Act. . . [Google Scholar]
- Moniveena M, Kumar T. An Overview of Track & Trace Regulations in Pharma Industry and its Impact on the Reverse Logistics of Medicines- Status in Regulated Countries and India. Int J Pharm Sci Rev Res. 2017;47(2):85-91. [Google Scholar]
- . Countdown to the 2017 DSCSA Deadline: Serialization Strategies for Compliance. . . [Google Scholar]
- Rotunno R, Cesarotti V, Bellman A, Introna V, Benedetti M. Impact of Track and Trace Integration on Pharmaceutical Production Systems. Int J Eng Business Manag. 2014;6. [Google Scholar] [Crossref]
- . CRB. (2015). Countdown to the 2017 DSCSA Deadline: Pharmaceutical Product Serialization. Regulations and Strategies for Compliance. . . [Google Scholar]
- . Cardinalhealth.com. n.d. HDA Guidelines For Bar Coding In the Pharmaceutical Supply Chain. . . [Google Scholar]
- Abstract
- Introduction
- Worldwide Regulatory Framework: History and Recent Scenario
- Serialization Blueprinting in Pharmaceutical Industry
- Serialization Barcoding for US FDA-Regulated Products
- Demonstration of two-dimensional (2D) Bar code in product label
- Demonstration of inner packs (Bundles)
- Demonstration of product case label (Homogeneous cases)
- Conclusion
- Source of Funding
- Conflict of Interest
- References
How to Cite This Article
Vancouver
Patel D, Singh DK, Patel B, Borkhataria C. Impact and overview of track and trace system and serialization in pharmaceutical industry [Internet]. Indian J Pharm Pharmacol. 2021 [cited 2025 Oct 21];8(1):47-51. Available from: https://doi.org/10.18231/j.ijpp.2021.008
APA
Patel, D., Singh, D. K., Patel, B., Borkhataria, C. (2021). Impact and overview of track and trace system and serialization in pharmaceutical industry. Indian J Pharm Pharmacol, 8(1), 47-51. https://doi.org/10.18231/j.ijpp.2021.008
MLA
Patel, Dhavalkumar, Singh, Dev Kumar, Patel, Birajuben, Borkhataria, Chetan. "Impact and overview of track and trace system and serialization in pharmaceutical industry." Indian J Pharm Pharmacol, vol. 8, no. 1, 2021, pp. 47-51. https://doi.org/10.18231/j.ijpp.2021.008
Chicago
Patel, D., Singh, D. K., Patel, B., Borkhataria, C.. "Impact and overview of track and trace system and serialization in pharmaceutical industry." Indian J Pharm Pharmacol 8, no. 1 (2021): 47-51. https://doi.org/10.18231/j.ijpp.2021.008
